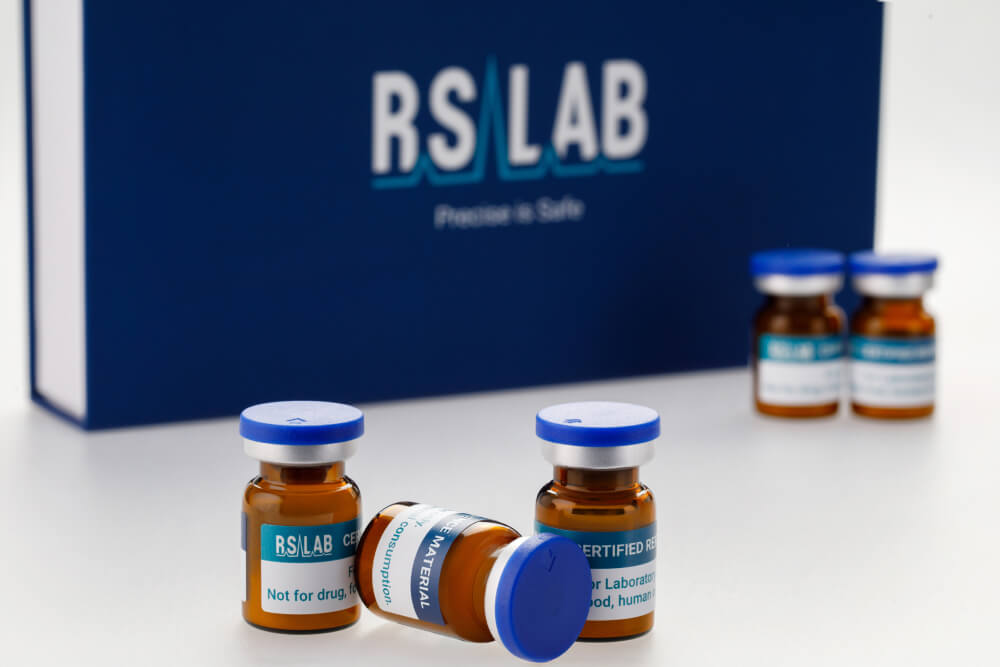




We drive precision and safety across industries.
At RefLabPharm, we set new standards in precision, safety, and compliance. Through innovation and expertise, we empower industries and researchers to drive progress and enhance global well-being.
Our Key Areas
- API & Impurity CRMs
- Custom CRM Solutions
- Analytical Services
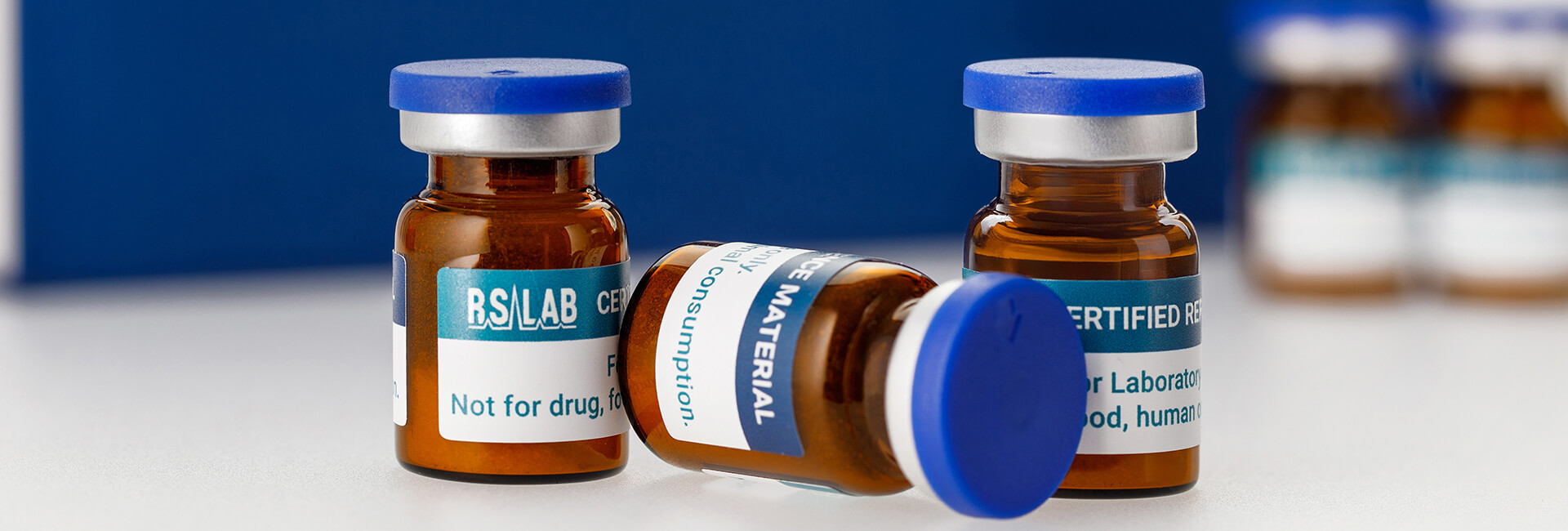
Our Products
RS-LAB API Certified Reference Materials (CRMs) ensure accuracy, reliability, and compliance in pharmaceutical analysis. Designed for API characterization, method validation, and quality control, our high-precision materials help industry professionals meet regulatory standards and maintain analytical accuracy.
RS-LAB Impurity Certified Reference Materials (CRMs) support impurity profiling, stability studies, and regulatory compliance. These materials enable our partners to detect, quantify, and control impurities, ensuring the safety and quality of pharmaceutical products.
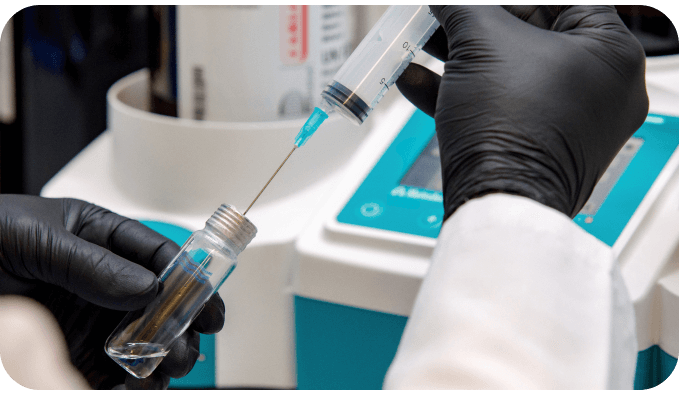
Our services
We offer specialized analytical services to support pharmaceutical development and regulatory compliance. Our expertise in stability testing, storage, and analytical development ensures precision, reliability, and quality control at every stage of the process. With a commitment to scientific excellence and industry standards, we help our partners achieve accurate, reproducible, and compliant results.
We provide comprehensive analytical development and testing to support method validation, impurity profiling, and quality control. Utilizing cutting-edge techniques, we ensure precise, reproducible, and compliant analytical results, helping our partners maintain regulatory standards and product integrity.
Our stability testing and storage services assess the shelf life, safety, and efficacy of pharmaceutical products under controlled environmental conditions. We conduct long-term, accelerated, and forced degradation studies to ensure product stability and compliance with ICH guidelines and regulatory requirements.
Contact us
Interested in one of our products or solutions? Connect with our experts to discuss your requirements. We provide optimal solutions tailored to your needs, ensuring precision, compliance, and reliability in every project.